Sunday, May 15, 2011
PERIODIC TABLE HISTORY
| Jöns Jakob Berzelius | 1828 | Developed a table of atomic weights. Introduced letters to symbolize elements. | ||||||
| Johann Döbereiner | 1829 | Developed 'triads', groups of 3 elements with similar properties. Lithium, sodium & potassium formed a triad. Calcium, strontium & barium formed a triad. Chlorine, bromine & iodine formed a triad. | Forerunner to the notion of groups. | |||||
| John Newlands | 1864 | The known elements (>60) were arranged in order of atomic weights and observed similarities between the first and ninth elements, the second and tenth elements etc. He proposed the 'Law of Octaves'. | Newlands' Law of Octaves identified many similarities amongst the elements, but also required similarities where none existed. He did not leave spaces for elements as yet undiscovered. Forerunner to the notion of periods. | |||||
| Lothar Meyer | 1869 | Compiled a Periodic Table of 56 elements based on the periodicity of properties such as molar volume when arranged in order of atomic weight. | Meyer & Mendeleev produced their Periodic Tables simultaneously. | |||||
| Dmitri Mendeleev | 1869 | Produced a table based on atomic weights but arranged 'periodically' with elements with similar properties under each other. Gaps were left for elements that were unknown at that time and their properties predicted (the elements were gallium, scandium and germanium). The order of elements was re-arranged if their properties dictated it, eg, tellerium is heavier than iodine but comes before it in the Periodic Table. | Mendeleev's Periodic Table was important because it enabled the properties of elements to be predicted by means of the 'periodic law': properties of the elements vary periodically with their atomic weights. | |||||
| William Ramsay | 1894 | Discovered the Noble Gases. | In 1894 Ramsay removed oxygen, nitrogen, water and carbon dioxide from a sample of air and was left with a gas 19 times heavier than hydrogen, very unreactive and with an unknown emission spectrum. He called this gas Argon. In 1895 he discovered helium as a decay product of uranium and matched it to the emission spectrum of an unknown element in the sun that was discovered in 1868. (helios is the Greek for Sun). He went on to discover neon, krypton and xenon, and realised these represented a new group in the Periodic Table. Ramsay was awarded a Nobel Prize in 1904. | |||||
| Henry Moseley | 1913 | Determined the atomic number of each of the elements. He modified the 'Periodic Law' to read that the properties of the elements vary periodically with their atomic numbers. | Moseley's modified Periodic Law puts the elements tellerium and iodine in the right order, as it does for argon and potassium, cobalt and nickel. | |||||
| 1914 | Predicted that there were 3 unknown elements between aluminium and gold and concluded there were only 92 elements up to and including uranium. | |||||||
ATOMIC HISTORY
Aristotle

Believed matter was made of different combinations of earth, air, fire, and water Lavoisier, A.L. (1743-1794)Discovered nitrogen; studied acids and described composition of many organic compounds.
Democritus

A greek philosopher who believed that atoms were indivisible spheres that could not be seen with the naked eye.
Lavoisier (late 1700's)

Stated earliest version of both the Law of Conservation and the Law of definite proportions.
Proust (1799)

Proved that Lavoisier's law by experiments
Dalton (1800's)

Defined atoms as solid and indestructible spheres based on the Law of Conservation of Mass
Thomson, (1856-1940)

Research on cathode rays resulted in proof of existence of electrons. Won the Nobel Prize in 1906
Rutherford (1871-1937):

Discovered that the atoms of the heavier elements, which had been thought to be irreversible, actually decay into various forms of radiation. Rutherford was the first to establish the theory of the nuclear atom and discovered rays: alpha, beta, and gamma. He also revealed the half-life of radioactive elements and applied this to studies of age determination of rocks by measuring the decay period of radium to lead-206.
This contradicts the plum pudding model by Thomson, which did not show a nucleus.
Next came Niels Bohr(1885-1962). He discovered that the electrons travel in discrete orbits around the nucleus in 1913.

The electrons from outer shell determine the property of atom. He inferred that when electrons move from outershells to an inner shell, it emits a quantum of energy(photon) in form of light.
He stated that electrons can only move in classical motions, not randomly.
He made contributions to the understanding of atomic structure and quantum mechanics.
Next came Henry Moseley (1887-1915). He made many contributions to the Periodic Table. He also performed experiments that proved that Bohr's model is actually valid. In 1913, he invented the Moseley's Law that concerns the characteristics of X-rays emitted by atoms.

It is important because it justifies the conception of nuclear model of the atom with all positive charges of atoms in the nucleus.
Before Moseley, the atomic number was just a place on the Periodic Table and was not associated with any measurable quantity.
Up untill now, scientist discovered the nucleus, electrons and the atom.
James Chadwick(1891-1974) discovered a previously unknown particles in the atomic nucleus. It was called neutron because of a lack of an electrical charge. This was crucial for fission of uranium-235. This made it possible to create elements heavier than uranium.


It is important because it justifies the conception of nuclear model of the atom with all positive charges of atoms in the nucleus.
Before Moseley, the atomic number was just a place on the Periodic Table and was not associated with any measurable quantity.
Up untill now, scientist discovered the nucleus, electrons and the atom.
James Chadwick(1891-1974) discovered a previously unknown particles in the atomic nucleus. It was called neutron because of a lack of an electrical charge. This was crucial for fission of uranium-235. This made it possible to create elements heavier than uranium.

Saturday, May 14, 2011
PERIODIC TABLE TRENDS
If you were to look carefully at many of the properties of the elements, you would notice something besides the similarity of the properties within the groups. You would notice that many of these properties change in a fairly regular fashion that is dependent on the position of the element in the periodic table. And of course, that is what you will do next. As you compare elements from left to right across the periodic table, you will notice a trend or regular change in a number of properties. The same thing happens if you go up and down on the periodic table and compare the properties of the elements.
You know that each atom has a nucleus inside and electrons zooming around outside the nucleus. It should seem reasonable that the size of an atom depends on how far away its outermost (valence) electrons are from the nucleus. If they are very close to the nucleus, the atom will be very small. If they are far away, the atom will be quite a bit larger. So the atomic size is determined by how much space the electrons take up.
1) As you move down a group, atomic radius increases.
Metals
Metalloids
Melting Point is when the element turns from solid to liquid, whereas the boiling point is when the element turns from liquid to gas. The graph pattern is fairly similar between the two, so we will discuss the melting point.

For each period the melting point rises from Group 1 to Group 14, then falls to the lowest value at Group 18. If the d-block elements are also included periodicity can be seen between rows of these elements, but as periodicity becomes less apparent with increasing atomic number this is less obvious than for the s- and p-block elements.
In other words, the melting and boiling points increase as we move accros a period, and increase as we move down a group.
Density can be simply defined as the mass of the atom divided by its volume.
1) It increases as you move accross a period, untill it reaches non-metals and metalloids where it starts decreasing again.
2) It increases as you move down a group.
The majority of metals have higher densities than the majority of nonmetals. The high density of most metals is due to the tightly packed crystal lattice of the metallic structure. The strength of metallic bonds for different metals reaches a maximum around the center of the transition metal series, as those elements have large amounts of delocalized electrons in tight binding type metallic bonds.
Atomic Radius
You know that each atom has a nucleus inside and electrons zooming around outside the nucleus. It should seem reasonable that the size of an atom depends on how far away its outermost (valence) electrons are from the nucleus. If they are very close to the nucleus, the atom will be very small. If they are far away, the atom will be quite a bit larger. So the atomic size is determined by how much space the electrons take up.
1) As you move down a group, atomic radius increases.
| WHY? - The number of energy levels increases as you move down a group as the number of electrons increases. Each subsequent energy level is further from the nucleus than the last. Therefore, the atomic radius increases as the group and energy levels increase. | ![[Image]](http://www.chem.tamu.edu/class/majors/tutorialnotefiles/Atomicradii.gif) |
2) As you move across a period, atomic radius decreases.
- WHY? - As you go across a period, electrons are added to the same energy level. At the same time, protons are being added to the nucleus. The concentration of more protons in the nucleus creates a "higher effective nuclear charge." In other words, there is a stronger force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius.
First Ionization Energy
Definition: The energy required to remove the outermost (highest energy) electron from a neutral atom in its ground state.1) As you move down a group, first ionization energy decreases.
- WHY?
- Electrons are further from the nucleus and thus easier to remove the outermost one. "SHIELDING" - Inner electrons at lower energy levels essentially block the protons' force of attraction toward the nucleus. It therefore becomes easier to remove the outer electron
WHY? - As you move across a period, the atomic radius decreases, that is, the atom is smaller. The outer electrons are closer to the nucleus and more strongly attracted to the center. Therefore, it becomes more difficult to remove the outermost electron.Exceptions to First Ionization Energy Trends
1) Xs2 > Xp1 e.g. 4Be > 5B
|
Second and Higher Ionization Energy
Definition: Second Ionization Energy is the energy required to remove a second outermost electron from a ground state atom.
Subsequent ionization energies increase greatly once an ion has reached the state like that of a noble gas. In other words, it becomes extremely difficult to remove an electron from an atom once it loses enough electrons to lose an entire energy level so that its valence shell is filled.
Na Mg Al | 495.8 737.7 577.6 | 4562.4 1450.6 1816.6 | 6912 7732.6 2744.7 | 9543 10,540 11,577 |
Metals, Non-Metals, and Metalloids
Metals
Common characteristics:Non-Metals
Metallic luster (shine)
Generally solids at room temperature
Malleable
Ductile
Conduct heat and electricity
Exist as extended planes of atoms
Combine with other metals to form alloys which have metallic characteristics
Form positive ions, e.g. Na+, Mg2+, and Al3
Common characteristics:
The differences in the characteristics of metals and nonmetals can be explained by the following:
Rarely have metallic luster (shine)
Generally gases at room temperature
Neither malleable nor ductile
Poor conductors of heat and electricity
Usually exist as molecules in thier elemental form
Combine with other nonmetals to form covalent
Generally form negative ions, e.g. Cl-, SO42-, and N3-
1) As you move across a period, metallic character decreases and nonmetallic character increases.
Metals have relatively few electrons in their valence shells.
Metals have lower ionization energies than nonmetals.
Metals have smaller electron affinities than nonmetals.
Metals have larger atoms than nonmetals.
2) As you move down a group, metallic character increases and nonmetallic character decreases.
Metalloids
A class of 8 elements that have properties of both metals and nonmetals.
| B | Si | Ge | As | Sb | Te | Po | At |
Common characteristics:
- Generally look metallic but are brittle (not malleable or ductile) Neither good conductors or insulators; instead they are semiconductors.
Electronegativity
- Electronegativity - The tendency of an atom to draw electrons in a bond toward itself.
- There are two periodic trends concerning electronegativity.
- As you move down a group, electronegativity decreases.
- As you move across a period, electronegativity increases.
Bonds can be classified according to the difference in electronegativities of the atoms (EN). Bonds are:
- ionic if
 EN > 1.8.
EN > 1.8. - polar covalent if 1.8

 EN
EN  0.4
0.4 - nonpolar covalent if
 EN < 0.4
EN < 0.4
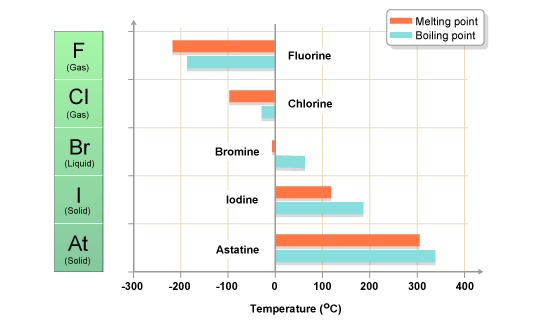
Melting and Boiling Points
Melting Point is when the element turns from solid to liquid, whereas the boiling point is when the element turns from liquid to gas. The graph pattern is fairly similar between the two, so we will discuss the melting point.

For each period the melting point rises from Group 1 to Group 14, then falls to the lowest value at Group 18. If the d-block elements are also included periodicity can be seen between rows of these elements, but as periodicity becomes less apparent with increasing atomic number this is less obvious than for the s- and p-block elements.
In other words, the melting and boiling points increase as we move accros a period, and increase as we move down a group.
Density
Density can be simply defined as the mass of the atom divided by its volume.
1) It increases as you move accross a period, untill it reaches non-metals and metalloids where it starts decreasing again.
2) It increases as you move down a group.
The majority of metals have higher densities than the majority of nonmetals. The high density of most metals is due to the tightly packed crystal lattice of the metallic structure. The strength of metallic bonds for different metals reaches a maximum around the center of the transition metal series, as those elements have large amounts of delocalized electrons in tight binding type metallic bonds.
PERIODIC TABLE FAMILIES
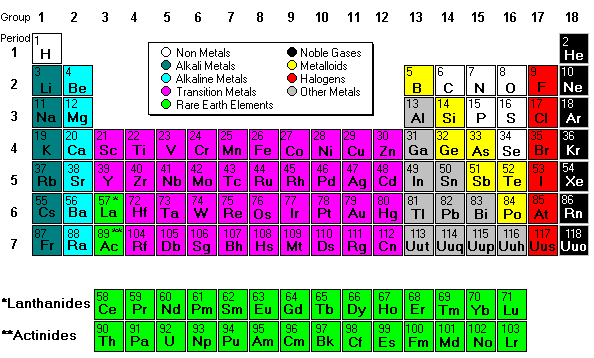
Mendeleev created the first periodic table based on atomic weight. He observed that many elements had similar properties, and that they occur periodically. Hence, the table’s name.
His periodic law states that the chemical and physical properties of the elements vary in a periodic way with their atomic weights. The modern one states that the properties vary with atomic number, not weight.
Elements in Mendeleev's table were arranged in rows called periods. The columns were called groups. Elements of each group had similar properties.
The Periodic table can be divided into nine families of elements each having similar properties. The families include:
Alkali metals
The alkali metals, found in group 1 of the periodic table, are highly reactive metals that do not occur freely in nature. These metals have only one electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements. As with all metals, the alkali metals are malleable, ductile, and are good conductors of heat and electricity. The alkali metals are softer than most other metals.

Alkaline metals
The alkaline earth elements are metallic elements found in the second group of the periodic table. All alkaline earth elements have an oxidation number of +2, making them very reactive.
The Transition metals
The 38 elements in groups 3 through 12 of the periodic table are called "transition metals." As with all metals, the transition elements are both ductile and malleable, and conduct electricity and heat. Their valence electrons are present in more than one shell. This is why they often exhibit several common oxidation states.

Other metals
The "other metals" elements are located in groups 13, 14, and 15. While these elements are ductile and malleable, they are not the same as the transition elements. These elements, unlike the transition elements, do not exhibit variable oxidation states, and their valence electrons are only present in their outer shell. All of these elements are solid, have a relatively high density, and are opaque. They have oxidation numbers of +3, ±4, and -3.
Metalloids
Metalloids are the elements found between the boundary that distinguishes metals from non-metals. Metalloids have properties of both metals and non-metals. Some of the metalloids, such as silicon and germanium, are semi-conductors.

Non-metals
Non-metals are the elements in groups 14-16 of the periodic table. Non-metals are not able to conduct electricity or heat very well. As opposed to metals, non-metallic elements are very brittle. The non-metals can be gases, such as oxygen and solids, such as carbon. The non-metals have no metallic luster, and do not reflect light. They have oxidation numbers of ±4, -3, and -2.
Halogens
The halogens are five non-metallic elements found in group 17 of the periodic table. All halogens have 7 electrons in their outer shells, giving them an oxidation number of -1.
Noble gases
The noble gases are found in group 18 of the periodic table. These elements have an oxidation number of 0. This prevents them from forming compounds readily. All noble gases have 8 electrons in their outer shell, making them stable.

Rare Earth
The 30 rare earth elements are composed of the lanthanide and actinide series. One element of the lanthanide series and most of the elements in the actinide series are synthetic, that is, human-made. All of the rare earth metals are found in group 3 of the periodic table, and the 6th and 7th periods.
VALENCE ELECTRONS
The valence electrons are the electrons in the last shell or energy level of an atom. They do show a repeating or periodic pattern. The valence electrons increase in number as you go across a period. Then when you start the new period, the number drops back down to one and starts increasing again.
A quick way to determine the number of valence electrons for a representative element is to look at which group is it in. Elements in group Ia have 1 valence electron. Elements in group IIa have 2 valence electrons. Can you guess how many valence electrons elements in group VIa have? If you guessed 6 valence electrons, then you are correct! The only group of representative elements that this method doesn't work for is group 0. Those elements certainly have more than 0 valence electrons; in fact, all of them except for helium have 8 valence electrons. Why doesn't helium have 8 valence electrons? Think for a moment about how many electrons helium has - it has a total of only two electrons, so helium only has 2 valence electrons.
So generally speaking, the number of valence electrons stays the same as you go up or down a group, but they increase as you go from left to right across the periodic table. The preceding statement works very well for the representative elements, but it comes a bit short of the truth when you start talking about the transition elements.
Electrons going into the d sublevels of the transition metals complicate this pattern. In some ways these electrons behave like valence electrons. In some other ways they behave like shielding electrons, which are discussed in the next section. The first electrons into a d sublevel seem to behave more like valence electrons but the last ones seem to act more like shielding electrons, with variations along the way. Switching the order from 4s3d to 3d4s is one way to represent this.
As it turns out, the idea of valence electrons is not very useful for transition metals, at least not in a reliable, predictable way.
| For example, when you go across the table from carbon to nitrogen to oxygen, the number of valence electrons increases from 4 to 5 to 6. As we go from fluorine to neon to sodium, the number of valence electrons increases from 7 to 8 and then drops down to 1 when we start the new period with sodium. Within a group--starting with carbon and going down to silicon and germanium--the number of valence electrons stays the same. |
|
So generally speaking, the number of valence electrons stays the same as you go up or down a group, but they increase as you go from left to right across the periodic table. The preceding statement works very well for the representative elements, but it comes a bit short of the truth when you start talking about the transition elements.
Electrons going into the d sublevels of the transition metals complicate this pattern. In some ways these electrons behave like valence electrons. In some other ways they behave like shielding electrons, which are discussed in the next section. The first electrons into a d sublevel seem to behave more like valence electrons but the last ones seem to act more like shielding electrons, with variations along the way. Switching the order from 4s3d to 3d4s is one way to represent this.
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | |
| outer configuration | 4s23d1 | 4s23d2 | 4s23d3 | 4s13d5 | 4s23d5 | 4s2 3d6 | 4s2 3d7 | 4s2 3d8 | 3d104s1 | 3d104s2 |
| apparent valence electrons | 3 | 2-4 | 2-5 | 2-6 | 2-7 | 2 or 3 | 2 or 3 | 2 or 3 | 1 or 2 | 2 |
ELECTRON CONFIGURATION
Key Concepts
- The number of electrons in an atom of an element corresponds to the element's Atomic Number (Z) and is equal to the number of protons in the nucleus of the atom.
H atom, Z = 1.
Number of protons in H atom = Z = 1
Number of electrons in H atom = Z = 1
- The number of electrons in the ion of an element corresponds to the element's Atomic Number (Z) minus the charge on the ion:
H+, Z = 1, charge = +1. Number of electrons in ion = Z - charge = 1 - (+1) = 0
H-, Z = 1 charge = -1. Number of electrons in ion = Z - charge = 1 - (-1) = 2
- Simple Electron Configuration shows the number of electrons in each energy level
- Lowest Energy levels are filled first
(ie, first energy level is filled first followed by the second then the third etc)
Energy Level Maximum Number of Electrons First 2 Second 8 Third 18 Fourth 32 - Filled energy levels correspond to the electron configuration of Group VIII elements
(the Noble or Inert Gases)
- Subshell Electron Configuration shows the number of electrons in each subshell
(or sub-level) within each energy level
Group of the Periodic Table subshell being filled Maximum Number of Electrons I and II s 2 III, IV, V, VI, VII, VIII p 6 Transition Metals d 10 Lanthanides and Actinides f 14 - Orbital Notation shows the number of electrons in each orbital within an energy level
Simple Electron Configuration
Maximum number of electrons in each energy level (shell):1st(K)=2, 2nd(L)=8, 3rd(M)=18, 4th(N)=32
The helium atom has 2 electrons in the first energy level.
Its simple electron configuration is 2
The neon atom has 10 electrons,
2 electrons in the first energy level and 8 electrons in the second energy level.
Its simple electron configuration is 2,8
The argon atom has 18 electrons,
2 electrons in the first energy level, 8 electrons in the second energy level, 8 electrons in the thrid energy level.
Its simple electron configuration is 2,8,8
| Period | Order for Filling Energy Levels | Energy Level | Shell | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | He | First | K | ||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | Second | L | ||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | Third | M | ||||||||||
| 4 | K | Ca | Transition Metals : third energy level filling | Ga | Ge | As | Se | Br | Kr | Fourth | N | |||||||||
| 5 | Rb | Sr | Transition Metals : fourth energy level filling | In | Sn | Sb | Te | I | Xe | Fifth | O | |||||||||
| 6 | Cs | Ba | Transition Metals : fifth energy level filling | Tl | Pb | Bi | Po | At | Rn | Sixth | P | |||||||||
| 7 | Fr | Ra | Transition Metals : sixth energy level filling | Seventh | Q | |||||||||||||||
| Lanthanides : fourth energy level filling | ||||||||||||||||||||
| Actinides : fifth energy level filling | ||||||||||||||||||||
Examples
| Atom | Z | No. Electrons = Z | Simple Electron Configuration | ||||
|---|---|---|---|---|---|---|---|
| H | 1 | 1 | 1 | ||||
| He | 2 | 2 | 2 | ||||
| Li | 3 | 3 | 2,1 | ||||
| Be | 4 | 4 | 2,2 | ||||
| B | 5 | 5 | 2,3 | ||||
| C | 6 | 6 | 2,4 | ||||
| N | 7 | 7 | 2,5 | ||||
| O | 8 | 8 | 2,6 | ||||
| F | 9 | 9 | 2,7 | ||||
| Ne | 10 | 10 | 2,8 | ||||
| Na | 11 | 11 | 2,8,1 | ||||
| Mg | 12 | 12 | 2,8,2 | ||||
| Al | 13 | 13 | 2,8,3 | ||||
| Si | 14 | 14 | 2,8,4 | ||||
| P | 15 | 15 | 2,8,5 | ||||
| S | 16 | 16 | 2,8,6 | ||||
| Cl | 17 | 17 | 2,8,7 | ||||
| Ar | 18 | 18 | 2,8,8 |
Sub-shell Electron Configuration
Order for filling subshells: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7pwhere the number indicates the energy level and the letter indicates the subshell being filled.
Maximum number of electrons in each subshell: s=2, p=6, d=10, f=14
A superscipt number after the letter of the subshell shows how many electrons occupy that subshell.
The helium atom has 2 electrons, both in the s subshell of the first energy level.
Its sub-shell electron configuration is 1s2
The neon atom has 10 electrons.
2 electrons in the s subshell of the first energy level: 1s2
8 electrons in second energy level, made up of 2 electrons in an s subshell and 6 electrons in a p subshell: 2s2 2p6
Its subshell electron configuration is 1s2 2s2 2p6
The argon atom has 18 electrons.
2 electrons in the s sub-shell of the first energy level: 1s2
8 electrons in the second energy level made up of 2 electrons in an s subshell and 6 electrons in a p subshell: 2s2 2p6
8 electrons in the third energy level made up of 2 electrons in an s subshell and 6 electrons in a p subshell: 3s2 3p6
Its subshell electron configuration is: 1s2 2s2 2p6 3s2 3p6
| Period | s subshell | d subshell | p subshell | Energy Level | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | He | First | ||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | Second | ||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | Third | ||||||||||
| 4 | K | Ca | Transition Metals : third energy level filling | Ga | Ge | As | Se | Br | Kr | Fourth | |||||||||
| 5 | Rb | Sr | Transition Metals : fourth energy level filling | In | Sn | Sb | Te | I | Xe | Fifth | |||||||||
| 6 | Cs | Ba | Transition Metals : fifth energy level filling | Tl | Pb | Bi | Po | At | Rn | Sixth | |||||||||
| 7 | Fr | Ra | Transition Metals : sixth energy level filling | Seventh | |||||||||||||||
| f subshell | Lanthanides : fourth energy level filling | ||||||||||||||||||
| f subshell | Actinides : fifth energy level filling | ||||||||||||||||||
Examples
| Ion | No. Electrons = Z - charge | Subshell Electron Configuration |
|---|---|---|
| H- | 1 - (-1) = 2 | 1s2 |
| Li+ | 3 - 1 = 2 | 1s2 |
| Be2+ | 4 - 2 = 2 | 1s2 |
| B3+ | 5 - 3 = 2 | 1s2 |
| C4+ | 6 - 4 = 2 | 1s2 |
| N3- | 7 - (-3) = 10 | 1s2 2s2 2p6 |
| O2- | 8 - (-2) = 10 | 1s2 2s2 2p6 |
| F- | 9 - (-1) = 10 | 1s2 2s2 2p6 |
| Na+ | 11 - 1 = 10 | 1s2 2s2 2p6 |
| Mg2+ | 12 - 2 = 10 | 1s2 2s2 2p6 |
| Al3+ | 13 - 3 = 10 | 1s2 2s2 2p6 |
| Si4+ | 14 - 4 = 10 | 1s2 2s2 2p6 |
| P3- | 15-(-3)=18 | 1s2 2s2 2p6 3s2 3p6 |
| S2- | 16-(-2)=18 | 1s2 2s2 2p6 3s2 3p6 |
| Cl- | 17-(-1)=18 | 1s2 2s2 2p6 3s2 3p6 |
| Atom | Z | No. Electrons = Z | Subshell Electron Configuration | |||
|---|---|---|---|---|---|---|
| H | 1 | 1 | 1s1 | |||
| He | 2 | 2 | 1s2 | |||
| Li | 3 | 3 | 1s2 2s1 | |||
| Be | 4 | 4 | 1s2 2s2 | |||
| B | 5 | 5 | 1s2 2s2 2p1 | |||
| C | 6 | 6 | 1s2 2s2 2p2 | |||
| N | 7 | 7 | 1s2 2s2 2p3 | |||
| O | 8 | 8 | 1s2 2s2 2p4 | |||
| F | 9 | 9 | 1s2 2s2 2p5 | |||
| Ne | 10 | 10 | 1s2 2s2 2p6 | |||
| Na | 11 | 11 | 1s2 2s2 2p6 3s1 | |||
| Mg | 12 | 12 | 1s2 2s2 2p6 3s2 | |||
| Al | 13 | 13 | 1s2 2s2 2p6 3s2 3p1 | |||
| Si | 14 | 14 | 1s2 2s2 2p6 3s2 3p2 | |||
| P | 15 | 15 | 1s2 2s2 2p6 3s2 3p3 | |||
| S | 16 | 16 | 1s2 2s2 2p6 3s2 3p4 | |||
| Cl | 17 | 17 | 1s2 2s2 2p6 3s2 3p5 | |||
| Ar | 18 | 18 | 1s2 2s2 2p6 3s2 3p6 |
Subscribe to:
Comments (Atom)
